Coronavirus disease (COVID-19) is caused by a new strain of Coronavirus (SARS-CoV-2) discovered in 2019 and not previously identified in humans. Common symptoms include fever, cough, and shortness of breath. On March 11, the World Health Organization (WHO) announced the current COVID-19 outbreak as a pandemic. Currently there are more than 681 million confirmed cases globally (data from March 9, 2023). According to data from Our World in Data, on March 9, 2023, 69.7% of the world population had received at least one dose of a COVID-19 vaccine, and 28% of people in low-income countries have received at least one dose.
What is currently known about COVID-19 and pregnancy
In May 2020, WHO reported there was no known difference between the clinical manifestations of COVID-19 in pregnant and non-pregnant women of reproductive age. The available data on the exact effects of COVID-19 on fertility and pregnancy remained scarce. Cochrane Gynaecology and Fertility joined the University of Birmingham and WHO to collaborate on the development of a living systematic review on COVID-19 in pregnancy. In March 2021, an updated version of the living systematic review and meta-analysis Clinical manifestations, risk factors, and maternal and perinatal outcomes of coronavirus disease 2019 in pregnancy was published in the BMJ. The updated review included 192 studies (64,676 pregnant women with COVID-19; 569,981 non-pregnant women with COVID-19). Results from the update showed that pregnant and recently pregnant women were more likely to need intensive care treatment for COVID-19 compared to non-pregnant women of reproductive age. Several risk factors for severe COVID-19 in pregnancy were identified - pre-existing comorbidities, non-white ethnicity, chronic hypertension, pre-existing diabetes, high maternal age, and high body mass index. Pregnant women with COVID-19 are more likely to deliver preterm compared to pregnant women without COVID-19. Babies born to mothers with COVID-19 are more likely to be admitted to the neonatal unit. On March 16, the living systematic review SARS-CoV-2 positivity in offspring and timing of mother-to-child transmission was published in the BMJ. The review included 472 studies (28 952 mothers, 18 237 babies). Evidence suggested confirmed vertical transmission of SARS-CoV-2, although this is likely to be rare. Severity of maternal covid-19 appears to be associated with SARS-CoV-2 positivity in offspring.
COVID-19 and pregnancy loss
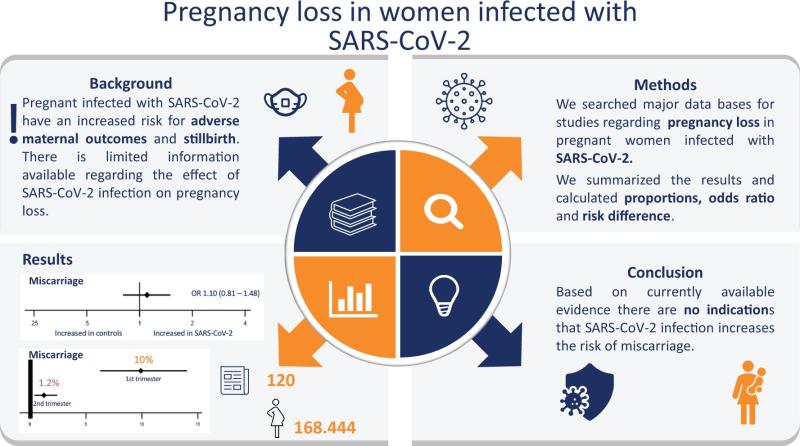
On November 28, 2023, we published our systematic review "COVID-19 in pregnant women: a systematic review and meta-analysis on the risk and prevalence of pregnancy loss" in Human Reproduction Update. Our study found no indication that SARS-CoV-2 infection in the first or second trimester increases the risk of miscarriages.
Resources on COVID-19, pregnancy, fertility, and vaccination
Here we have gathered important resources, guidance and advice statements from international organizations and societies related to COVID-19, fertility and pregnancy. We are working on updating the information below as soon as new guidelines become available.
We have also created an Excel sheet that contains data extracted from all published reports on pregnancy and neonatal outcomes in women with confirmed COVID-19. Please note that this data table has not been peer reviewed. If you have unpublished data on pregnancy and neonatal outcomes in women with confirmed COVID-19 from your institute, and you would like us to include it in the database, please contact dr Madelon van Wely (m.vanwely@amsterdamumc.nl).
Update: as of March 2023, the database will not be updated on a regular basis. However, we plan to resume our activities if needed.
Download Excel sheet Perinatal outcomes in COVID-19 infection.
Image:CDC
On March 14, 2020, as a precautionary measure ESHRE advised that “all fertility patients considering or planning treatment, even if they do not meet the diagnostic criteria for COVID-19 infection, should avoid becoming pregnant at this time”. On October 14, 2020, ESHRE released a new guidance Safe ART services during the third phase of the COVID-19 pandemic. On February 25, 2022, ESHRE published an updated statement on SARS-COV-2 and assisted reproduction. With regards to vaccination, ESHRE recommends that, if available, men and women attempting to conceive through assisted reproduction receive the COVID-19 vaccine before starting treatment or at any time during the fertility treatment or pregnancy. You can download all guidance documents and statements from the COVID-19 and ART dedicated page.
On March 17, 2020, ASRM started publishing guidance documents on fertility care during the COVID-19 pandemic. Read all updates here: Patient Management and Clinical Recommendations During The Coronavirus (COVID-19) Pandemic. In January, 2021, SART and ASRM published FAQs for Patients Related to COVID-19. On April 20, 2022, ASRM published their latest ASRM COVD-19 Task Force Issues Update No. 20, stating physicians and health care providers should continue to discuss and encourage vaccination and booster shots with all patients who are considering pregnancy or who are currently pregnant. For all updates visit ASRM's COVID-19 Updates and Resources page.
On May 11, 2020, HFEA issued General Direction GD0014 (version 2) that requires clinics to have a written COVID-19 Treatment Commencement Strategy before resuming licensed treatments. Direction 0014 was revoked by the Authority on 18 May, 2022. On April 20, 2022, HFEA updated their COVID-19 professional guidance stating HFEA licensed clinics have incorporated safe ways of working for patients and clinic staff. For all guidance resources and updates visit Coronavirus (COVID-19) and fertility treatment.
On May 6, 2020, British Fertility Society & Association of Reproductive & Clinical Scientists released a U.K. best practice guidelines for reintroduction of routine fertility treatments during the COVID-19 pandemic. On January 13, 2022, BFS published an updated FAQs on Covid 19 Vaccines & Fertility. On February 28, 2022, BFS and ARCS published an update to the best practice guidelines for fertility clinics during the COVID-19 pandemic.
NVOG has developed a flowchart and FAQ related to COVID-19 and pregnancy (information in Dutch). On January 6, 2022, NVOG published an updated statement about vaccination and pregnancy (in Dutch) underscoring the importance that all pregnant women should get vaccinated (with an mRNA vaccine) and receive a booster dose. The statement was updated on September 9, 2022. For all publications and updates go the dedicated COVID-19 page.
On March 7, 2022, RCOG published an updated guideline for healthcare professionals on coronavirus (COVID-19) infection in pregnancy. On April 16, 2021, the Joint Committee on Vaccination and Immunisation (JCVI) announced it will be offering COVID-19 vaccination to all pregnant women (with the Pfizer-BioNTech or Moderna mRNA vaccine). For all updates go to Coronavirus (COVID-19), pregnancy and women’s health and the dedicated page on COVID-19 vaccination and pregnancy.
More information on COVID-19 and vaccination can be found here: COVID-19 Vaccines While Pregnant or Breastfeeding and COVID-19 Vaccines for People Who Would Like to Have a Baby.
On December 21, 2020, ACOG published a COVID-19 Vaccination Considerations for Obstetric–Gynecologic Care (last updated September 20, 2022). ACOG recommends that all eligible persons aged 6 months and older, including pregnant and lactating individuals, receive a COVID-19 vaccine or vaccine series. For all resources and updates visit the COVID-19 dedicated page.
On March 24, 2020, FSA published an updated statement recommending that “in the interest of public safety, patients who are planning to start fertility treatment consult with their treating specialist and discuss the appropriateness of postponing their treatment.” On April 4, 2020, the society published a COVID-19 FAQs page.
On March 12, 2020, ISUOG published Interim Guidance - 2019 novel coronavirus infection during pregnancy and puerperium: information for healthcare professionals. An update of the guidance was published on May 1, 2020. For all updates and information for patients, visit COVID-19 resources page. For updates visit Patient Information: COVID-19 and pregnancy.
INSPQ published COVID-19: Interim Recommendations on Preventive Workplace Measures for Pregnant and Nursing Workers in Community transmission context. For all updates and statements go to INSPQ COVID-19 dedicated page and the dedicated COVID-19 vaccination and immunization page.
